A-Class® Highly Cross-Linked Polyethylene
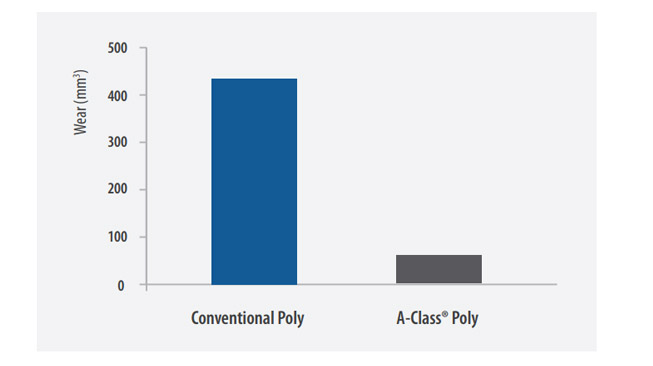
The balanced processing of A-Class® Highly Cross-Linked Polyethylene drastically reduces wear while maintaining the material's mechanical properties to create a superior bearing surface.
Poly material selection
Specific polyethylene has been chosen to manufacture the acetabular liners with a high impact strength, tensile strength, and yield strength.1
Cross-linking process
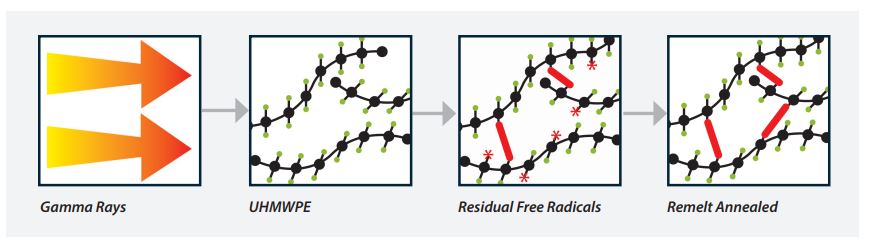
The selected polyethylene is subjected to a radiation cycle to facilitate cross-linking of the polymers and enhance wear resistance. The mechanical properties of the material are maintained.
Heat treatment
Through a proprietary thermal remelting cycle, the rods are then heated above the melting point of the polyethylene to eliminate residual free radicals, form additional cross-links, and improve the oxidative stability of the material.2
Machining & final sterilization
Liners are machined, cleaned, packaged, and sterilized using ETO sterilization, which does not reintroduce free radicals or cause any other measurable change to the polymer.